Stroke has become the first cause of death and disability in China, among which ischemic stroke accounts for 60% ~ 70%. Clinical tissue plasminogen activator (tPA) thrombolysis and mechanical thrombectomy are two magic weapons for vascular recanalization of ischemic stroke. Due to the high technical requirements of mechanical thrombectomy for equipment and personnel, intravenous thrombolysis is still the main treatment for recanalization of blood vessels after stroke. In clinical practice, tPA thrombolytic therapy has shown double-edged sword effect: it can effectively dissolve thrombus only within 4.5 hours of time window, and significantly increase the risk of cerebral hemorrhage in patients after thrombolysis, so only 3% of stroke patients benefit.
After acute ischemic stroke, ischemic penumbra is formed in the ischemic penumbra around the infarct core, which is the main brain tissue that can be saved by acute recanalization therapy. The ischemic penumbra usually survives for only a few hours, which is the main cause of thrombolytic time window stenosis. Previous researchers suggested that the integrity of the blood-brain barrier in ischemic areas of brain tissue after stroke was damaged, and after recanalization of blood vessels, blood components from the damaged blood-brain barrier entered the brain parenchyma, causing haemorrhagic transformation. But the exact mechanism of hemorrhagic transformation is not clear. It is of great theoretical and practical significance to elucidate the mechanism of the tPA thrombolytic window for stenosis after stroke and the increased risk of bleeding after thrombolysis, so that more patients will benefit from tPA treatment.
To solve these problems, professor ShiFuDong team has established the tPA intravenous thrombolysis for acute ischemic stroke treatment of prospective study cohort, the longitudinal comparison of the 71 patients who underwent tPA intravenous thrombolytic therapy in patients with acute cerebral apoplexy, the change of peripheral immune cells before and after thrombolysis, found that tPA rapidly increase the number of peripheral blood neutrophils and lymphocytes. To clarify the mechanism and its impact on the transformation of bleeding after thrombolysis, teams use thromboembolic stroke model in rats had in-depth exploration, found that tPA by acting on expression of annexin A2 immune cells, regulate the transcriptome gene expression of these cells, quickly activated peripheral immune cells, and thus increase the blood-brain barrier damage, promoting the transformation of bleeding after thrombolysis. Inhibition of neutrophil chemotaxis by THE CCL2 inhibitor Bindarit or inhibition of peripheral lymphocyte migration by the sphygin-1-phosphate receptor (S1PR) regulator significantly reduces the risk of thrombolysis after cerebral embolization and improves neurological function (Figure 1). This study, published online in Circulation Research on October 19, 2020, confirms that precise immune intervention may reduce the risk of bleeding transformation in patients with ischemic stroke who receive thrombolytic therapy, and provides a new treatment strategy to solve this clinical problem.
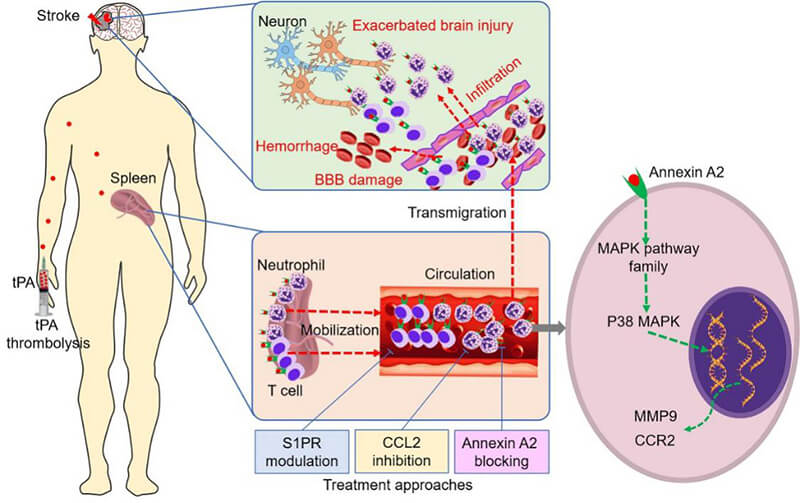
Figure 1. Immune mechanism and intervention strategies of haemorrhagic transformation after tPA thrombolysis.
TPA thrombolysis rapidly activates and mobilizes peripheral neutrophils and T cells to migrate to ischemic brain tissue, aggravating cerebrovascular inflammation, destruction of the blood-brain barrier and haemorrhagic transformation. TPA binds to the immune cell's Annexin A2 and activates the intracellular MAPK signaling pathway, leading to upregulation of matrix metalloproteinase 9 (MMP9) and C-C chemokine receptor 2 (CCR2). Immune interventions targeting neutrophilic chemokines and sphingomyelin-1-phosphate receptor (S1PR) can reduce the activation of immune cells by tPA by inhibiting the migration of immune cells, thus reducing the destruction of the blood-brain barrier and post-thrombolytic hemorrhage transformation (Circulation Research 2020).
The study is a continuation of Professor Shi's team's exploration of immune intervention as a combination therapy for acute stroke. Since 2014, two clinical studies have demonstrated that Fingolimod, an immunomodulator, combined with tPA thrombolytic therapy for acute ischemic stroke significantly reduced thrombolytic transformation and extended the thrombolytic time window. As an oral immunomodulator, Fingomod prevents the migration of lymphocytes from the secondary lymphoid organs to the periphery, thus reducing their intracranial infiltration. Two clinical proof-of-concept studies and a number of preclinical studies conducted by Professor Shi's team have confirmed that Fingomide can inhibit intracranial inflammation and reduce secondary brain injury in stroke patients (PNAS 2014, JAMA Neurology 2014). Within 4.5 hours of the onset of acute ischemic stroke, combined treatment with fingomide and tPA was safe and feasible, and reduced patients' hemorrhagic transformation (Circulation 2015), laying a foundation for the in-depth mechanism research mentioned above. Fingomod can also extend the thrombolytic window of tPA to 6 hours (Annals of Neurology 2018) by reducing vascular inflammation after stroke, promoting cerebral microcirculation perfusion and collateral circulation establishment, and protecting the ischemic penumbral zone. The study was named Wiley's most downloaded article of the year 2018-2019 (Figure 2).

FIG. 2. The 2018 study on thrombolytic window expansion in acute ischemic stroke by Shi Fudong's team combined with immune intervention and tPA was the most downloaded paper of the year by WILEY Press.
"Ask canal which get so clear, to have source running water to come". In the decade from 2010 to 2020, Professor Shi's team has completed a series of pioneering explorations in the organic integration of clinical and basic research in terms of the immune mechanism and immune intervention of stroke. The series of research results became the basis for the team to win the first prize of Tianjin Natural Science, two Chinese Stroke Awards and market transformation (Figure 3). In the acute phase of stroke, stroke immunology arises at the historic moment, which provides a new perspective and clue for the pathogenesis of stroke, and also lays a theoretical foundation for the exploration of immune intervention as a single drug or combined drug in the treatment of stroke, and brings a new light of treatment.
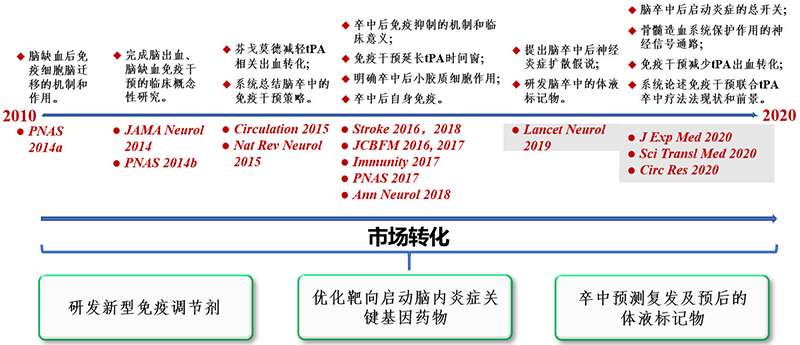
Figure 3. Immunological studies and transformations of stroke: a ten-year journey.

Figure 4. Key members who completed the work (from left to right) Shi Kaibin, Jia Dongmei, Shi Fudong and Zou Ming, at Tianjin General Hospital on October 19, 2020.
References